Core technologies leading to the future
Utilizing our technology in production of nucleic acid-based umami seasoning, we line up high-quality nucleic related compounds. These are used in broad fields such as pharmaceutical, cosmetic, and other industrial products.
Chemical and enzymatic modifications of nucleotides and nucleosides
Manufacturing various natural materials, APIs and pharmaceutical intermediates by chemical synthesis and/or enzymatic synthesis using nucleotides/nucleosides prepared by the RNA degradation method
Chemical modifications of phosphorylated compounds
Synthesizing not only nucleic acid related compounds but also nucleobase derivatives and phosphorylated compounds by utilizing synthetic and purification technologies for nucleic acid related compounds
Synthesis and purification of hydrophilic compounds
Supplying hydrophilic products with high purity based on high technologies of chromatography and crystallization of hydrophilic compounds
Modification technologies
We manufacture APIs of small-molecule drugs related to nucleic acids. In addition to the listed products which are examples of APIs for generic drugs, we are manufacturing other APIs.
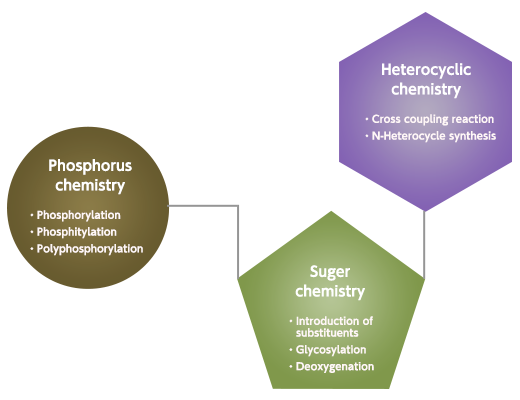
Followings are Yamasa's core modification technologies for the nucleotide compounds, nucleobase, five-carbon sugar and phosphate group.
Nucleobase : Cross coupling reaction, N-heterocycle synthesis
Sugar : Introduction of various substituents, deoxygenation, glycosylation
Phosphate group : Phosphorylation, phosphitylation, polyphosphorylation
In addition, other reactions are available regardless of compounds.
| Reaction name | Representative |
|---|---|
| Acylation | Acylation of hydroxyl group by acylchloride or acid anhydride etc |
| Amidation | Amidation of carboxylic acid derivatives by ammonia or other amines |
| Amination | Substitution of aryl halide by amines, reduction of nitro or nitroso group |
| Alkylation | Alylation of hydroxyl group by alkyl halide |
| Esterification | Condensation of organic acids (or phosphoric acids) and alcohols |
| Cyanylation | Dehydration of amides or oximes |
| Halogenation | Reaction of unsaturated compounds with halogenation reagents (e.g. NBS, NCS), substitution of organosulfate derivatives by halides |
| Oxidation | Oxidation of alcohols into carbonyl-compounds |
| Reduction | Reduction of carbonyl-compounds into alcohols |
| Glycosylation | Glycosylation catalyzed by Lewis acids |
Related Links
Inquiry by e-mail
Contact by e-mailInquiry by telephone
(Reception hours 9:00~17:30)